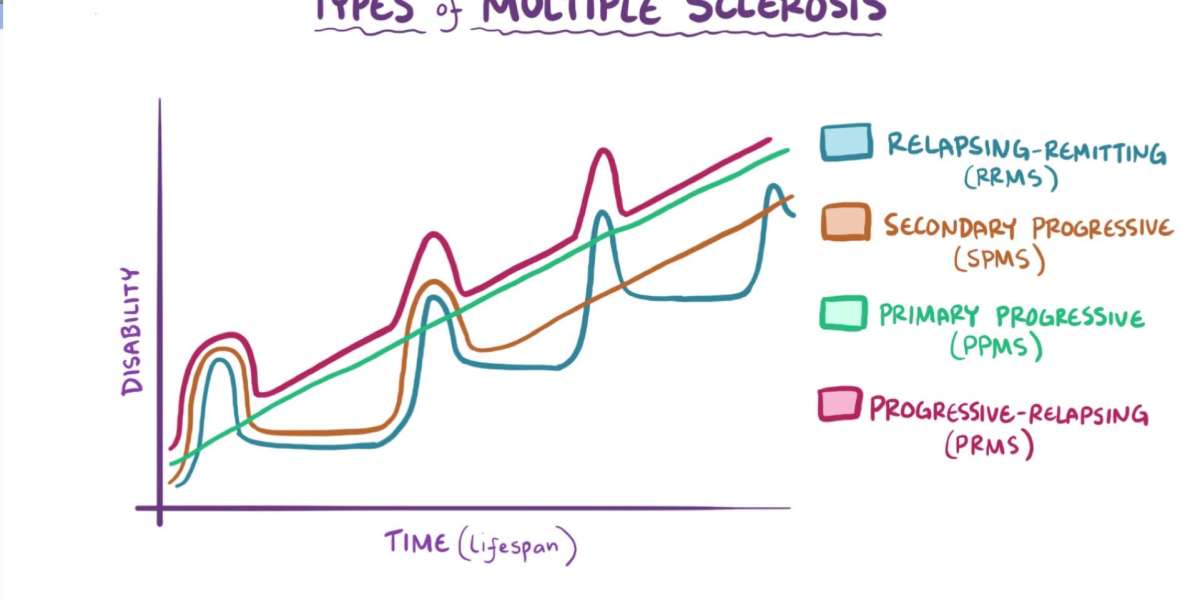
The Primary Progressive Multiple Sclerosis (PPMS) market is undergoing a notable transformation, driven by therapeutic breakthroughs, enhanced disease understanding, and a pressing need to address unmet clinical demands. PPMS, a rare and debilitating form of multiple sclerosis (MS), is characterized by a continuous progression of neurological deterioration without distinct periods of remission. Representing approximately 15% of all MS cases globally, PPMS significantly impacts patient quality of life and imposes a considerable burden on healthcare systems. Unlike relapsing-remitting multiple sclerosis (RRMS), which is more widely studied and treated, PPMS remains a major clinical challenge, but recent developments are reshaping the landscape of this specialized neurodegenerative disease market.
For insights into the emerging trends and market dynamics shaping the future of Primary Progressive Multiple Sclerosis care, explore our in-depth analysis of Primary Progressive Multiple Sclerosis treatment market insights.
Primary Progressive Multiple Sclerosis Treatment Market Overview
The Primary Progressive Multiple Sclerosis treatment market is expected to witness robust growth in the coming years, supported by a combination of increasing disease prevalence, improved diagnostic capabilities, and the introduction of disease-modifying therapies (DMTs) tailored specifically to PPMS. Traditionally, most approved MS therapies have been geared towards relapsing forms, leaving a significant therapeutic void for PPMS patients. However, the approval of Ocrelizumab for Primary Progressive Multiple Sclerosis—the first and only FDA-approved therapy for PPMS—has marked a pivotal shift in clinical practice. This monoclonal antibody targets CD20-positive B cells, validating B-cell depletion as a viable therapeutic strategy and encouraging further research into similar mechanisms. Pharmaceutical companies are increasingly investing in advanced therapeutic options, spurred by the success of ocrelizumab and growing regulatory support for orphan drug development in rare neurological conditions.
Primary Progressive Multiple Sclerosis Epidemiological Trends
From an epidemiological standpoint, Primary Progressive Multiple Sclerosis is showing gradual growth in incidence, partially attributed to improved diagnostic protocols and aging populations in high-income countries. Globally, more than 3 million people live with MS, with PPMS accounting for nearly 450,000 of these cases. Primary Progressive Multiple Sclerosis prevalence tends to be higher in North America and Europe, where advanced imaging and biomarker-based diagnostics enable earlier and more accurate detection. In contrast, developing regions continue to experience underdiagnosis, largely due to limited access to magnetic resonance imaging (MRI) and neurological specialists. Additionally, PPMS typically presents in individuals aged 40 to 60, aligning with demographic trends in aging populations across Western countries—another key factor driving future growth in this segment of the neurodegenerative disease market.
For further insights and detailed research on Primary Progressive Multiple Sclerosis Epidemiology, visit the Primary Progressive Multiple Sclerosis patient pool.
Primary Progressive Multiple Sclerosis Market Drivers
Multiple Primary Progressive Multiple Sclerosis market drivers are fueling momentum in research and commercialization. Rising awareness among healthcare professionals and patients has led to earlier interventions and an expanding base of diagnosed cases. The therapeutic validation of B-cell-targeted agents has also prompted a broader exploration of immune-modulating pathways. This has encouraged pipeline diversification, with pharmaceutical and biotech firms focusing on agents that not only modulate immune responses but also offer neuroprotective and remyelinating potential. The increasing use of real-world evidence (RWE), wearables, and digital health platforms is optimizing both clinical trial designs and long-term disease monitoring. Furthermore, incentives provided by regulatory agencies for orphan drug designation have accelerated research and development timelines, enabling companies to bring innovative therapies to market more efficiently.
Primary Progressive Multiple Sclerosis Unmet Needs and Challenges
Despite these advancements, the Primary Progressive Multiple Sclerosis market still faces significant barriers. A substantial proportion—estimated at around 40%—of patients with non-active PPMS remain untreated, primarily due to the lack of effective therapies for more advanced or inactive disease stages. Even among those receiving treatment, disability often progresses, underscoring the urgent need for neuroprotective agents that go beyond immunomodulation. Additionally, the high cost of DMTs like ocrelizumab presents a formidable challenge, especially in healthcare systems with limited reimbursement frameworks. Clinicians frequently cite the management of non-motor symptoms such as cognitive decline, depression, and fatigue as critical unmet needs in patient care. The lack of validated biomarkers for treatment response continues to hinder the application of personalized medicine approaches, creating a gap between clinical research and real-world patient management.
Primary Progressive Multiple Sclerosis Competitive Landscape and Pipeline Innovations
The Primary Progressive Multiple Sclerosis competitive landscape is rapidly evolving, with a growing number of companies pursuing novel mechanisms of action. Bruton tyrosine kinase (BTK) inhibitors such as tolebrutinib and evobrutinib are gaining attention for their dual immunomodulatory effects on peripheral B cells and central nervous system (CNS) microglia. Stem cell-based interventions, including mesenchymal stem cell therapies under development by companies like ImStem Biotechnology, are exploring avenues for both immunoregulation and tissue repair. Nanocatalytic agents like CNM-Au8, which target mitochondrial function and remyelination, represent a new frontier in neurotherapeutics. Meanwhile, Roche continues to refine the use of ocrelizumab, with its subcutaneous formulation demonstrating non-inferiority to intravenous administration in Phase III trials—offering patients a more convenient route of administration and potentially improving adherence rates. Emerging players such as MediciNova are also contributing to a dynamic Primary Progressive Multiple Sclerosis pipeline analysis, investigating immunomodulatory vaccines and small molecule therapies aimed at reducing inflammation and slowing disease progression.
For detailed insights on emerging therapies and trends within the Primary Progressive Multiple Sclerosis treatment market, download the full report.
Future Directions in Primary Progressive Multiple Sclerosis Treatment
Looking ahead, the future of the Primary Progressive Multiple Sclerosis market will likely be shaped by continued innovation, strategic collaborations, and the integration of precision medicine frameworks. Biomarker discovery will be instrumental in enabling more individualized treatment pathways, while expanded access to genetic and proteomic profiling could pave the way for truly personalized therapeutic regimens. The incorporation of artificial intelligence and machine learning into clinical decision-making and trial optimization also holds promise for accelerating drug development and improving patient outcomes. As the field moves from bench to bedside, ensuring equitable access to these novel therapies will be essential to realizing their full clinical and commercial potential.
Conclusion
In conclusion, the Primary Progressive Multiple Sclerosis market is at a critical juncture, transitioning from a historically underserved segment to a focus of intense research and investment. Breakthroughs in treatment such as ocrelizumab have catalyzed a shift in clinical expectations, while emerging innovations in immunology, neurology, and regenerative medicine are poised to further transform the standard of care. Although challenges remain—particularly in addressing late-stage disease and economic disparities—the collective progress in Primary Progressive Multiple Sclerosis treatment, epidemiology, and drug development provides a solid foundation for sustained market expansion and improved patient care in the years to come.
For further insights and detailed updates on this evolving field, visit our comprehensive insights and expert analysis.
Read More
- Primary Progressive Multiple Sclerosis Pipeline Insight
- Primary Progressive Multiple Sclerosis Epidemiology Forecast
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve their performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.